Vascular and pigmented neurocristopathies
Team Leader
H. ETCHEVERS
H. ETCHEVERS
Our group studies how certain kinds of birth defects occur. Those affecting derivatives of the stem cell population known as the neural crest are called congenital neurocristopathies. The palate, eyes and/or heart are frequently affected, but dozens of rarer diseases present also symptoms in the skin, gut or the nervous system. While the genetic bases of many specific neurocristopathies over the last decade have been found, most remain without molecular diagnosis and are relatively poorly defined as clinical entities due to their diverse presentations and rarity.
We study a subset of malformation syndromes due to mutations also found in many adult cancers. Such mutations lead to constant activation of normally temperorarily active enzymes in only some cell types and can be lethal, depending on when they occur. The result in survivors is inappropriate growth factor signaling, leading to effects on cell identities and tissue growth. The resulting organism is a mosaic of affected (mutated) and unaffected (non-mutated) cells. Their interactions during development can lead to diseases that appear very different from, but are mechanistically related to and sometimes predispose to, certain cancers.
Mouse models used by our group phenocopy (reproduce many aspects of) syndromes found in human fetuses or children by directing the same types of mutations to distinct multipotent neural crest derivatives. This can affect not only their progeny in the skin but also in the heart, the peripheral nervous system, the pituitary or the skull. Characterizing these models and searching for equivalent mutations in relevant patient cohorts should lead to improved diagnoses and new therapeutic approaches for congenital neurocristopathies by repurposing drugs used in targeted chemotherapies.
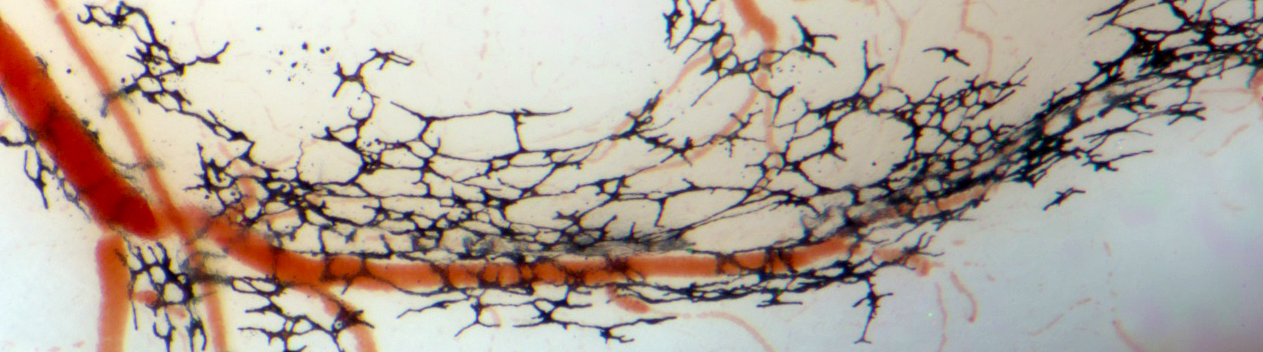
The large and giant congenital melanocytic nevus (CMN) is a visibly conspicuous malformation of the skin, present at birth. It can present as a restricted, stable and benign tumor, or be associated in syndromic form with additional cutaneous, neurological or oncological symptoms.
We study the effects of the molecular signaling pathways shown to be present in CMN in the embryological precursors to pigment cells using multiple systems:
We have successfully recruited a new postdoctoral colleague for 2024, Dr. Daniel Aldea, to carry this project forward as part of the EU-funded MELCAYA consortium.
Somatic mutations in genes encoding one or more effectors of the RAS-RAF and PI3K signaling pathways, and propagated in the cellular components of blood vessels, cause rare, defiguring and sometimes lethal vascular malformations.
Our transgenic mouse and human cellular models for the expression of such mutations in neural crest lineages implicates these signaling pathways not only in vascular but also craniofacial malformations affecting the skull and palate, in progressive peripheral neuropathies, and in neuroendocrine disorders.
This national consortium project launched just before the COVID-19 pandemic with the Zaffran group. It entails mapping the specific transcriptomic signatures of each cell in the developing human body in order to better understand normal and disease-associated physiology during prenatal life. We are participating in technology development with the GBiM platform as well as contributing unprecedently detailed data on the cellular composition of the first-trimester heart as it turns from a primordium into a functional and vital organ. This data contributes to the international Human Cell Atlas effort. Other tissues are also in the pipeline, in local collaborations and with further support from the AFM Téléthon and INSERM.